5th October
Polymorphism of carbon ,phosphorus and sulphur
Polymorphism can be defined as ability of a solid material to exist in more than one form or crystal structure.This usually occurs in different chemical compounds this is a little different from allotropy as allotropy is the existence of two or more different physical forms of a chemical element and this obviously as the definition says occurs in chemical elements.
Let us start off with talking about an element which has a very special place in chemistry and an element which whose amazing properties let to creation of new branches in chemistry.
Yes we are indeed talking about carbon.
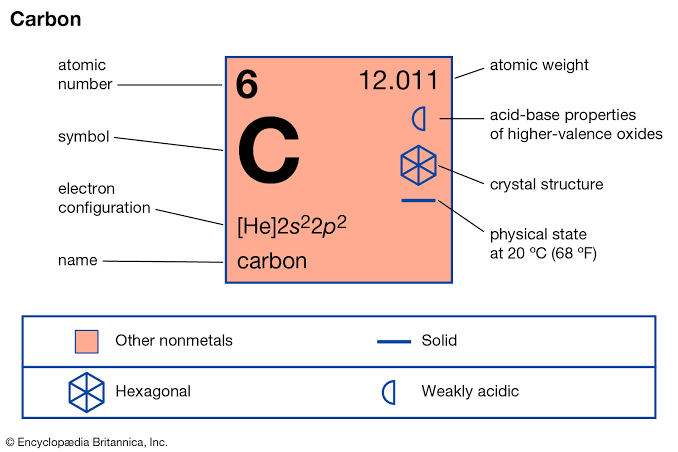
Carbon is a member of group 14 of in the periodic table . It’s a non-metal with 4 valence electrons . An important property of carbon that makes it so different from other elements is catenation this enables one carbon to form bonds with other carbon atoms generating ring and chain like structures in the process .
Carbon forms gaint covalent structures including diamond and graphite .It also forms Nanotubes and fullerenes.
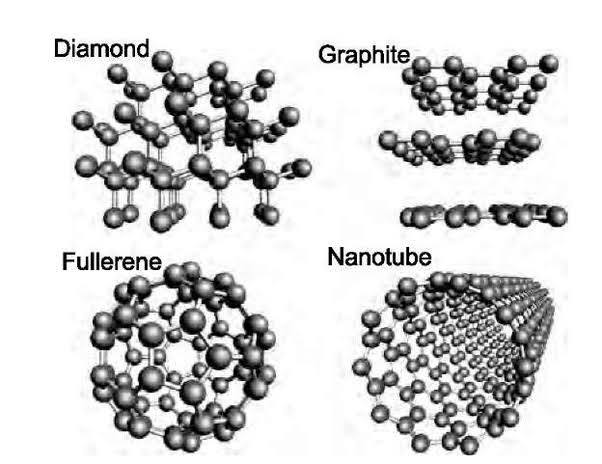
Diamond

Structure and bonding

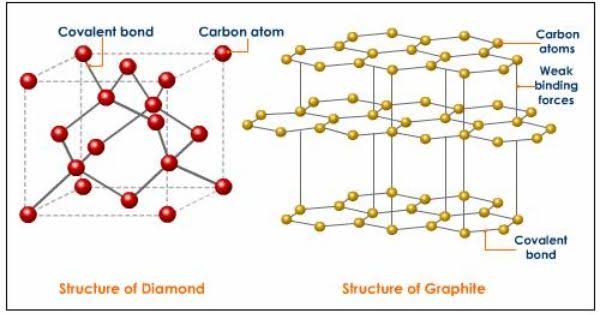
• Every C in diamond is sp3 hybridized and makes four covalent bonds with other carbon atoms.
• The carbon atoms have a regular lattice arrangement
• There are no free electrons in the structure. • Carbon atom has a tetrahedral arrangement in the structure.
This tetrahedral arrangment of C atoms give rise to sphalerite like cubic structure.
In this structure Carbon is present in
• All the corners of the cube
• All the face centers
• And 4 carbons arrannged tetrahedrally within the cube.
Then no of C atoms per unit cell = (1/8)*8+(6*(1/2))+4=8
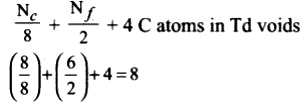
Physical properties and uses
• A three dimensional network of C atoms with strong covalent bonds makes diamond extremely hard.It is as a matter of fact one of the hardest substances to exist on earth’s surface.
• Diamond does not conduct electricity as there are no free electrons available all the electrons are tightly bound by the atoms .
• Is insoluble in water and organic solvents as there is no possible attractions that could occur between the solvent and carbon.
• High density (3.51 g/cm3)
Due to its hardness diamond is used in cutting tools like diamond tipped glass cutters and oil rig drills.
Graphite

Structure and bonding
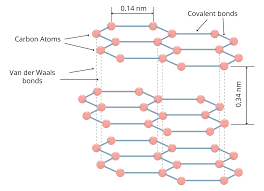
In the structure of graphite
• Each C is sp2 hydrised and the sheet
contains hexagonal rings.Each carbon is bound to only three other carbon atoms
• The C atoms form layers with hexagonal arrangement of atoms.
• The layers have weak Vanderwal’s forces between them
• Each C atom has a non-bonded outer electron which is delocalized and thus acts as a free electrons and allows conduction of electricity,
as they are free to move about anywhere within the sheet
• Graphite is a two dimensional arrangment of C atoms
Note1:The delocalized electrons are free to move anywhere within the particular sheet but there is no direct contact between delocalized electrons of one sheet and delocalized electrons of another sheet .
Note 2:Within a sheet the C atoms are held together by strong covalent bonds even stronger than diamond due to additional bonding caused by the delocalized electrons.
9 October 2020
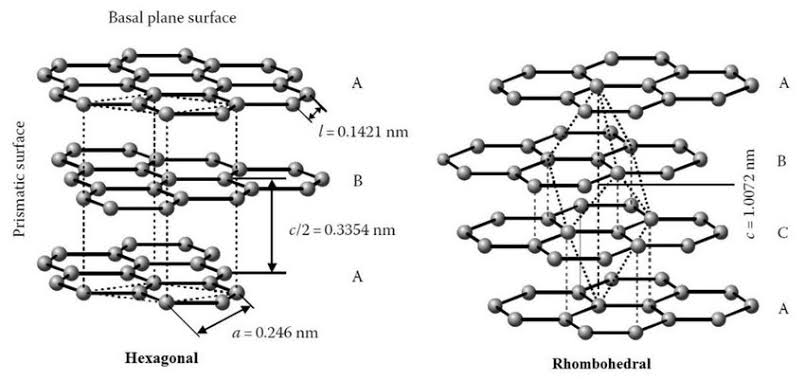
Graphite is found in two different forms α(hexagonal form) and β (rhombohedral form)
In hexagonal form layers are stacked in ABAB pattern such that the C atoms of the alternate layers are vertically above on the other hand in the rhombohedral form the layers are stacked in ABCABC fashion.
Physical properties of graphite
• Graphite too like diamond is insoluble in water and other organic solvents for the same reason as in diamond.
• Graphite conducts electricity as delocalized electrons are free to move about anywhere within the sheet.
• Graphite too has a high melting point.
• It is soft and slippery unlike diamond.
• Has a lower density than diamond due to higher amount of space wasted between the sheets.
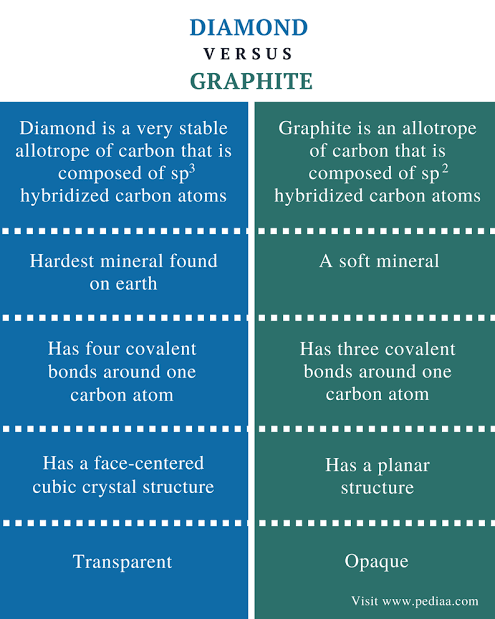
Comparison between diamond and graphite
Trivia
Diamonds are forever is a saying which we often hear but it isn’t exactly true as at higher temperatures or under intense ion bombardment, diamond degrades to graphite .
Also note that since diamond is made out of carbon, diamonds can burn just like coal. Therefore, if enough oxygen is present, diamond at high temperature will combust to form carbon dioxide .
