Inductive effect
Inductive effect is a permanent effect in which the charge density shifts to the more electronegative element which in turn results in formation of partial positive charges and negative charges in a molecule.
The effect is bought about by electron withdrawing groups(EWG) and electron donating groups(EDG),they bring about -I and +I effect respectively.
Enhancement of acid strength
An EWG enhances the strength of an acid.
While an EDG reduces the strength of an acid.
Let us consider different substituted acetic acids then the order of there acid strengths are as follows
FCH2COOH>ClCH2COOH>CH3COOH
Although both F and Cl are EWGs as F is more electronegative than Cl it is a much better EWG and enhances the acid strength better than Cl.
Also in another example we can see
(MeO)CH2COOH>(MeS)CH2COOH>(Me)COOH
Here as O is more electronegative than S ,(MeO) CH2COOH is a better acid.
Enhancement of basicity
EDG enhances the basicity or strength of of a base while EWG would reduce it.
Going into examples of some substituted ammonia componds
we see the trend of basic strength is as follows
NMe3>NH3>NF3
Clearly NMe3 is a much stronger base due to the presence of three methyl groups attached to N.
While the basicity decreasing as the groups attached to N show more and more electron withdrawing tendencies.
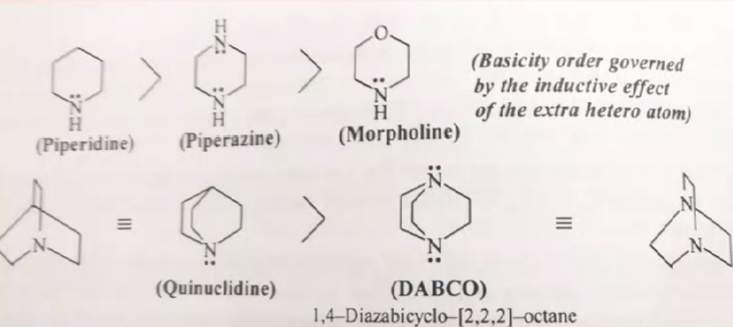
Even the the above examples
morpholine and DABCO is the least basis due to the presence of a more electronegative oxygen and nitrogen respectively which are essentially EWGs here.
Effect of hybridization
The decreasing order of acid strength for various states of hybridization of C is as follows
HC≡CH (sp- C)>H₂C=CH₂(sp2-C)>H3C-CH3(sp3-C)
While increasing order of basic strength is as follows
HC≡CH (sp C)<H₂C=CH₂ (sp2-C)<H3C-CH3(sp3 C)
An easier way to remember can be the molecules with more s character has more acid strength(or lesser basic strengths)
For example we have
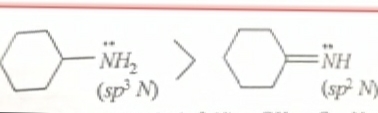
The only difference between the two structures in example one is the double bond and the hybridisation clearly the one with least s character(sp3) is more basic