Drago-Wayland Equation

This equation predicts the strength of acid-base interactions ,-ΔH.

In this theory the strength of acids and bases are explained in terms of oxide ions .
Here acids are oxide ion acceptors while bases are oxide ion donors.
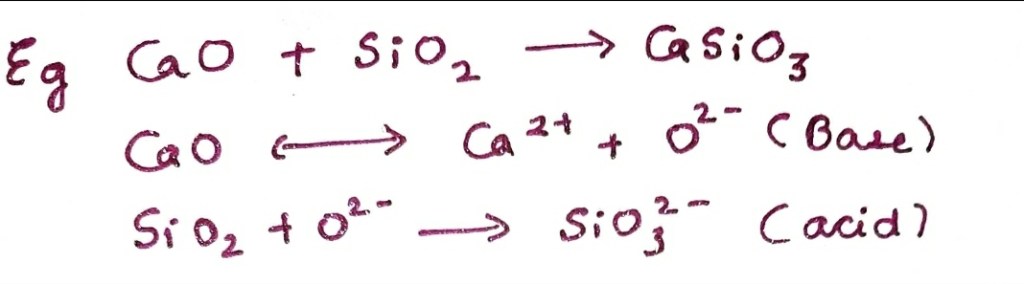
Here CaO behaves as a base by donating oxide ion while SiO₂ acts as an acid by accepting oxide ion.
This concept includes acids that act as non-protic acids and bases.
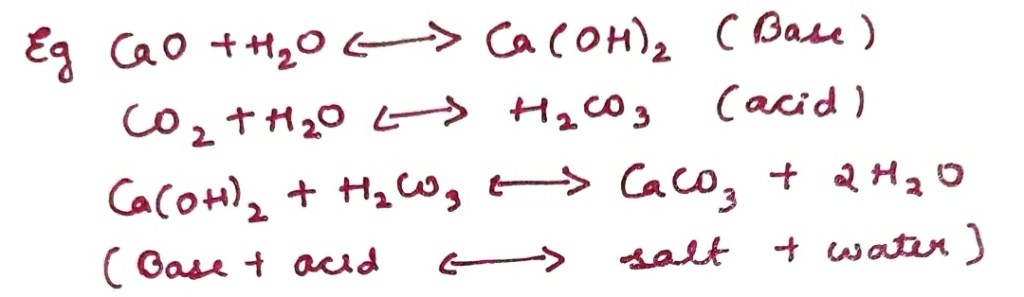
Iriving-Williams Series
This was one of the first theories than explored the trends in stability of metal complexes .
This theory explained how certain ligands formed stable complexes with heavy metal ions like Ag2+,Hg2+ etc.
While other ligands formed stable complexes with light metal ions like Co3+,Al3+ etc.
Thus metal ions were made into two classes
Class A metal ions are generally small , light and have a high charge density
They included alkali metals, alkaline earth metals and lighter transition metals of higher oxidation states like Ti4+,Cr3+ etc
While Class B metal ions are bigger , heavier and their charge density is more diffused(hence not very high charge density).They included heavier transition metals and those in lower oxidation states such as Cu+,Ag+,Hg+ etc
Ligands were thus classified into two groups based on which type of metal ions they form a stable complex with
Type A
They have a tendency to form complexes with class A metals ions.
Type B
They have a tendency to form complexes with class B metal ions.
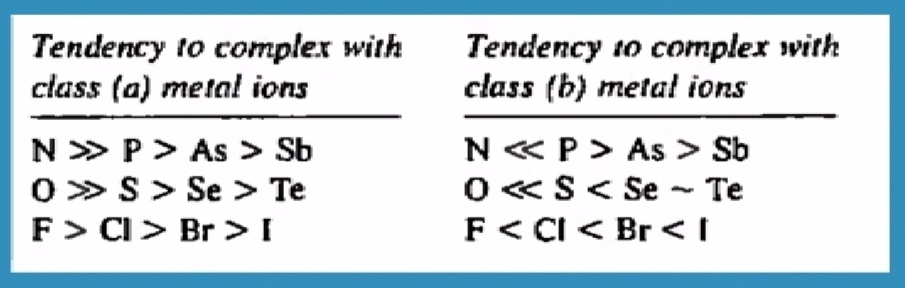
From the above table we see clearly that how smaller ,lighter
ligands prefer class A metal ions and heavier and bigger ligands prefer to form complexes with class B metal ions.
Pearson’s principle
Pearson extended Iriving-Williams theory .He suggested the names hard and soft to class A and class B compounds . Thus a hard acid is a class A metal ion and hard base is the type A ligand and similarily class B metal ions are soft acids and there corresponding type B ligands are soft bases.