Properties of hard acids and bases • Small atomic/ionic radius
• High oxidation state
• Low polarizability
• High electronegativity(for bases)
• HOMO of hard bases are of low energy
• LUMO of hard acids are of high energy
The affinity towards each other in case of hard acids and bases are ionic in nature.
Some general examples:
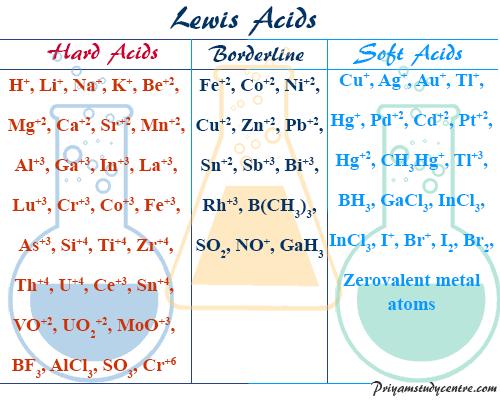
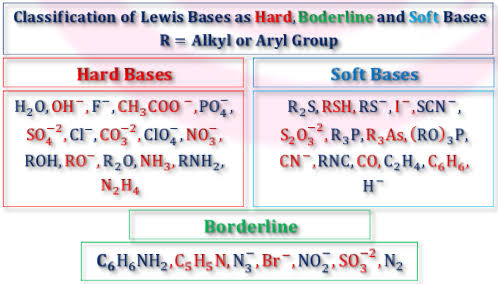
For hard acids:Ti4⁺,Cr3⁺,H⁺
For hard bases:OH-,F-,NH3 etc
Properties of soft acids and bases
• Large atomic/ionic radius.
• Low or zero oxidation state.
• High polarizability
• Low electronegativity
• HOMO of soft bases are of higher energy than hard bases.
• LUMO of soft acids are of lower energy than hard acids.
• The affinity towards each other in case of soft acids and bases are covalent in nature.
• Some general examples:
For soft acids:Hg+,Au+,Ag+
For soft bases:H-,I-,SCN-
Stability of complexes
A hard metal interacts with a hard base similar way to that of a proton hence the stability of a hard acid-base complex increases with increase in the basicity of the base.
One such example is :Given a choice between water and ammonia a metal ion will bind to ammonia preferentially as it is more basic.
In case of a soft acid-base interaction we know they are largely covalent and most metal ions have partially or fully filled d orbitals this emphasizes the importance of metal to ligand pi bonding in these complexes and hence they are much stabler than predicted by electrostatic arguments.
Borderline cases
Clearly as we have mentioned several times for acids and bases the terms like hard and soft are relative that is ,there is no sharp dividing line between hard and soft which creates a third category for acids and bases this category is called borderline cases. Consider the case of hard acids itself here Cesium ions and litium metal ions both belong to this hard acid category however lithium metal ions are harder than the Cesium ions (due to the large size of Cesium ions and high polarizability compared to lithium ions).Similar observations are found in case of bases as well .
Exceptions in HSAB theory or Violation of the theory
It is possible for a strong acid or base to displace a weaker one although the HSAB theory will be violated here.
Consider example one sulfite ion is a softer base while HF is a hard acid ,still they interact to give a hard base fluoride ion.This is because of the fact that the soft base sulfite ion is a very strong base and it displaces HF to give you a weak base F-.
In example 2 ,the strong hard base OH- interacts with soft acid methylmercury cation to displace a weaker soft base sulfite ion.
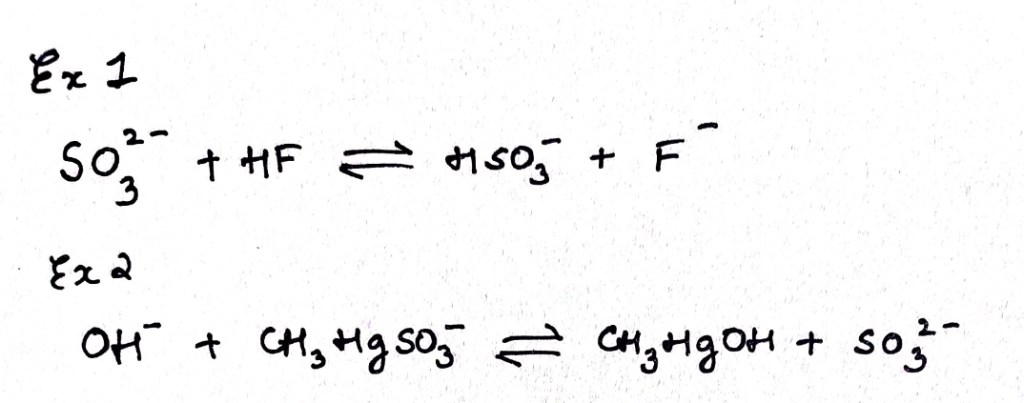
In both these cases the strength of the bases forced the reactions to the right or made them happened dispite the hard soft considerations, however when a competative environment is set up in which both strength and hardness-softness is considered the hard soft rule works
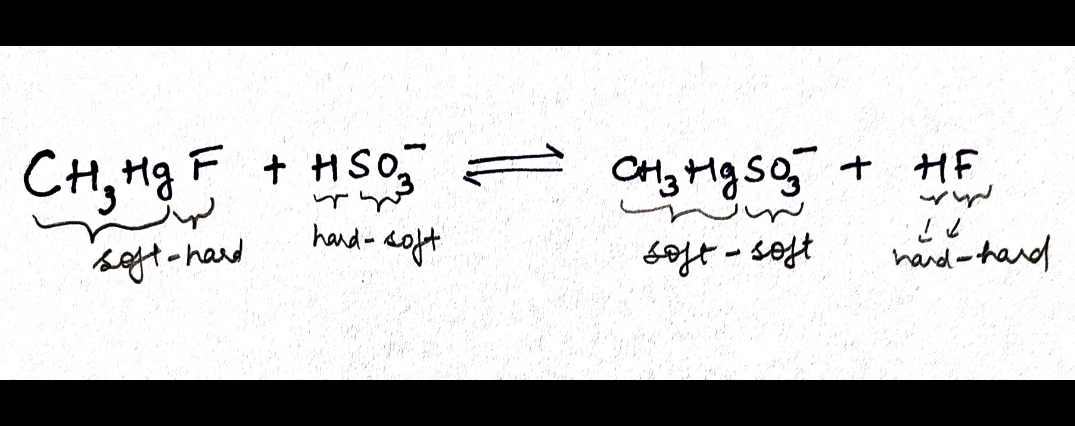
As illustrated in the example below